There are no products in your shopping cart.
| 0 Items | $0.00 |
info@cognistat.com | +1 (514) 336 0136
There are no products in your shopping cart.
| 0 Items | $0.00 |
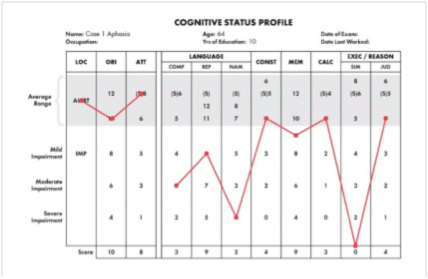
1987: The first of over 400 peer-reviewed papers was published in the Annals of Internal Medicine.
https://annals.org/aim/article-abstract/702185/
https://www.ncbi.nlm.nih.gov/pubmed/3631786
1988 – 2017: Cognistat has been translated into Spanish, French, Japanese, Hindi, Mandarin, Cantonese, Arabic, Czech, Swedish, Norwegian, Finnish, Hebrew, etc.
2009: Cognistat was adapted for computer use with the development of the Cognistat Assessment System (CAS). This is a web-based computer-assisted version of Cognistat by providing clinicians with electronic medical records, longitudinal patient data and test administration guidance and advice. It is an exact copy of the original paper and pencil test. CAS is clinician administered so that the existing body of validations studies remains intact.
2012: Cognistat Active Form was developed as an adjunct screening instrument for nurse practitioners, military users and clinicians who needed an electronic version of Cognistat but lacked access to the internet. It was designed to be run on a laptop without the need for web connectivity.
2014: Cognistat Five was developed to fulfill the need for a rapid (i.e. 5 minute) screen for delirium, mild cognitive impairment (MCI) and Alzheimer’s disease. It uses a subset of the original Cognistat subtests and includes a new expert system for risk assessment of MCI called the MCI Index. It is provided in three formats - a paper test, an non-web based computerized test and a fully web-based system.
2017: CAS-II, a new and more sophisticated version of the Cognistat Assessment System was introduced. CAS-II offers a new complex algorithm-based expert system for alerting the clinician to concerns during test administration and for highlighting significant patterns of cognitive decline in the test data.